| Abstract
- Stevia leaves are an important source of natural sugar substitute.
There are some restrictions on the use of stevia extract because of its
distinctive aftertaste. Some authors attribute this to soluble material
other than the stevia glycosides, even though it is well known that
stevia glycosides have to some extent a bitter taste. Therefore, the
purpose of this work was to develop a process to obtain stevia extract
of a better quality. The proposed process includes two steps: i)
Pretreatment of the leaves by SCFE; ii) Extraction of the stevia
glycosides by SCFE using CO2 as solvent and water and/or ethanol as
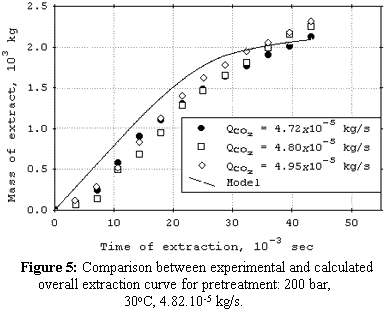
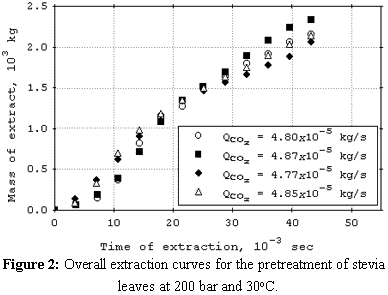
cosolvent. The mean total yield for SCFE pretreatment was 3.0%. The yields for SCFE with cosolvent of stevia glycosides were below 0.50%, except at 120 bar, 16°C, and 9.5% (molar) of water. Under this condition, total yield was 3.4%. The quality of the glycosidic fraction with respect to its capacity as sweetener was better for the SCFE extract as compared to extract obtained by the conventional process. The overall extraction curves were well described by the Lack extended model. |


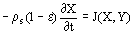 (1)
(1) 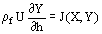 (2)
(2) 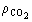 , can adequately
be used as rf.
, can adequately
be used as rf.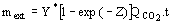 (3)
(3) 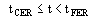
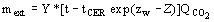 (4)
(4) 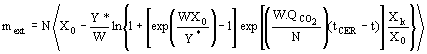 (5)
(5) 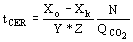 (6)
(6) 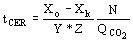 (7)
(7) 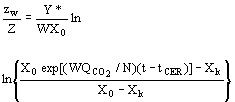 (8)
(8) 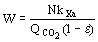 (9)
(9) 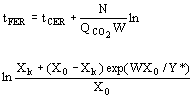 (10)
(10) 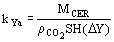 (11)
(11) 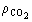 the solvent density (kg/m3), and D Y the average
logarithmic mean for the solute mass ratio in the fluid phase (Ferreira
et al., 1999):
the solvent density (kg/m3), and D Y the average
logarithmic mean for the solute mass ratio in the fluid phase (Ferreira
et al., 1999):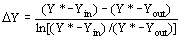 (12)
(12) 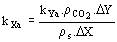 (13)
(13) 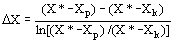 (14)
(14)